Please choose your shipping country in order to get correct prices and freight cost.
Africa
America
Asia
Europe
Oceania
-
Africa
- Algeria
- Angola
- Benin
- Botswana
- Burkina Faso
- Burundi
- Cameroon
- Cap Verde
- Central African Republic
- Chad
- Comoros
- Congo, Dem. Rep.
- Congo, Repub. of the
- Cote d'Ivoire
- Djibouti
- Egypt
- Equatorial Guinea
- Eritrea
- Ethiopia
- Gabon
- Gambia
- Ghana
- Guinea
- Guinea Bissau
- Kenya
- Lesotho
- Liberia
- Libya
- Madagascar
- Malawi
- Mali
- Mauritania
- Mauritius
- Mayotte
- Morocco
- Mozambique
- Namibia
- Niger
- Nigeria
- Reunion
- Rwanda
- Saint Helena
- Sao Tome & Principe
- Senegal
- Seychelles
- Sierra Leone
- Somalia
- South Africa
- Sudan
- Swaziland
- Tanzania
- Togo
- Tunisia
- Uganda
- Western Sahara
- Yemen
- Zambia
- Zimbabwe
-
America
- Anguilla
- Antigua & Barbuda
- Argentina
- Aruba
- Bahamas
- Barbados
- Belize
- Bermuda
- Bolivia
- Brazil
- British Virgin Is.
- Canada
- Cayman Islands
- Chile
- Colombia
- Costa Rica
- Cuba
- Dominica
- Dominican Republic
- Ecuador
- El Salvador
- French Guiana
- Grenada
- Guadeloupe
- Guatemala
- Guyana
- Haiti
- Honduras
- Jamaica
- Martinique
- Mexico
- Montserrat
- Netherlands Antilles
- Nicaragua
- Panama
- Paraguay
- Peru
- Puerto Rico
- Saint Kitts & Nevis
- Saint Lucia
- Saint Vincent & the Grenadines
- St Pierre & Miquelon
- Suriname
- Trinidad & Tobago
- Turks & Caicos Is
- United States
- Uruguay
- Venezuela
- Virgin Islands
-
Asia
- Afghanistan
- Armenia
- Azerbaijan
- Bahrain
- Bangladesh
- Bhutan
- Brunei
- Burma
- Cambodia
- China
- East Timor
- Gaza Strip
- Georgia
- Hong Kong
- India
- Indonesia
- Iran
- Iraq
- Israel
- Japan
- Kazakhstan
- Korea, North
- Korea, South
- Kuwait
- Kyrgyzstan
- Laos
- Lebanon
- Macau
- Malaysia
- Maldives
- Mongolia
- Nepal
- Oman
- Pakistan
- Philippines
- Qatar
- Saudi Arabia
- Singapore
- Sri Lanka
- Syria
- Taiwan
- Tajikistan
- Thailand
- Turkmenistan
- United Arab Emirates
- Uzbekistan
- Vietnam
- Yemen
-
Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Gibraltar
- Greece
- Greenland
- Guernsey
- Hungary
- Iceland
- Ireland
- Isle of Man
- Italy
- Jersey
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Netherlands
- Norway
- Poland
- Portugal
- Romania
- Russia
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Oceania
- Home
- Products
-
Products
- Pharmaceutical Quality Dextran
- Dextran 1 (EP/USP)
- Dextran 1.5
- Dextran 3.5
- Dextran 5
- Dextran 10
- Dextran 20
- Dextran 25
- Dextran 40 (EP/USP/JP)
- Dextran 60 (EP)
- Dextran 70 (EP/USP/JP)
- Dextran 110
- Dextran 150
- Dextran 250
- Dextran 500
- Technical Quality Dextran
- Dextran T1
- Dextran T1.5
- Dextran T3.5
- Dextran T5
- Dextran T6
- Dextran T10
- Dextran T20
- Dextran T25
- Dextran T40
- Dextran T60
- Dextran T70
- Dextran T110
- Dextran T150
- Dextran T250
- Dextran T500
- Dextran T750
- Dextran Derivatives
- DEAE-Dextran 500
- DEAE-Dextran 500 Pharmaceutical Quality
- Dextran Ultra
- Dextran 10
- Dextran 20
- Dextran 40
- Dextran 70
- Dextran 500
- Dextran Standards & GPC Standards
- Dextran Kit
- Dextran 1
- Dextran 5
- Dextran 12
- Dextran 25
- Dextran 50
- Dextran 80
- Dextran 150
- Dextran 270
- Dextran 410
- Dextran 670
- Dextran 2000
- Dextran 3800
- Dextran 5400
- Application Areas
- about dextran
- PentaHibe®
Carboxymethyl-Dextran
Pharmacosmos Carboxymethyl-Dextran (CM-Dextran) are based on Pharmacosmos’ world-leading Dextran products, and Pharmacosmos CM-Dextran are also produced at Pharmacosmos’ state-of-the-art, GMP-approved facility in Denmark.
As a natural consequence of Pharmacosmos’ commitment to quality, Pharmacosmos CM-Dextran are developed and manufactured according to the same advanced standards and requirements of the highest quality as applied in the development and manufacturing of our world-leading Dextran products.
CM-Dextran
CM-Dextran is made by reacting dextran with monochloric acid under alkaline conditions. The reaction is illustrated in Fig. 1 below. CM-Dextran is a polyanion in which the content of carboxyl groups is in the range 3% - 10%; corresponding to one carboxyl group for every 2.5 glucose units or less. The pKa value of the carboxyl group is approximately 8.2.
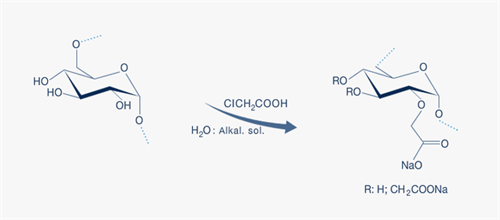
Fig. 1: CM-Dextran

Contact Specialist
Anne-Marie J. Ørkild
Director, International Sales and Business Carbohydrates
Phone: +45 5948 5959
E-mail: dextran@pharmacosmos.com
Approved
Pharmacosmos is the only dextran manufacturer holding certificates from the US FDA and European Directorate for the Quality of Medicines (EDQM)
Quality Delivered
Pharmacosmos delivers a unique dextran quality through a production technology that completely avoids the use of organic solvents and a quality system that meets the strictest cGMP standards for human medicines
Global Service

Pharmacosmos sells and ships directly to clients everywhere in the World. We deliver Pharmaceutical Quality Dextran of the highest standards, including those of the European Pharmacopoeia (EP), the United States Pharmacopoeia (USP), and the Japanese Pharmacopoeia (JP).

