Please choose your shipping country in order to get correct prices and freight cost.
Africa
America
Asia
Europe
Oceania
-
Africa
- Algeria
- Angola
- Benin
- Botswana
- Burkina Faso
- Burundi
- Cameroon
- Cap Verde
- Central African Republic
- Chad
- Comoros
- Congo, Dem. Rep.
- Congo, Repub. of the
- Cote d'Ivoire
- Djibouti
- Egypt
- Equatorial Guinea
- Eritrea
- Ethiopia
- Gabon
- Gambia
- Ghana
- Guinea
- Guinea Bissau
- Kenya
- Lesotho
- Liberia
- Libya
- Madagascar
- Malawi
- Mali
- Mauritania
- Mauritius
- Mayotte
- Morocco
- Mozambique
- Namibia
- Niger
- Nigeria
- Reunion
- Rwanda
- Saint Helena
- Sao Tome & Principe
- Senegal
- Seychelles
- Sierra Leone
- Somalia
- South Africa
- Sudan
- Swaziland
- Tanzania
- Togo
- Tunisia
- Uganda
- Western Sahara
- Yemen
- Zambia
- Zimbabwe
-
America
- Anguilla
- Antigua & Barbuda
- Argentina
- Aruba
- Bahamas
- Barbados
- Belize
- Bermuda
- Bolivia
- Brazil
- British Virgin Is.
- Canada
- Cayman Islands
- Chile
- Colombia
- Costa Rica
- Cuba
- Dominica
- Dominican Republic
- Ecuador
- El Salvador
- French Guiana
- Grenada
- Guadeloupe
- Guatemala
- Guyana
- Haiti
- Honduras
- Jamaica
- Martinique
- Mexico
- Montserrat
- Netherlands Antilles
- Nicaragua
- Panama
- Paraguay
- Peru
- Puerto Rico
- Saint Kitts & Nevis
- Saint Lucia
- Saint Vincent & the Grenadines
- St Pierre & Miquelon
- Suriname
- Trinidad & Tobago
- Turks & Caicos Is
- United States
- Uruguay
- Venezuela
- Virgin Islands
-
Asia
- Afghanistan
- Armenia
- Azerbaijan
- Bahrain
- Bangladesh
- Bhutan
- Brunei
- Burma
- Cambodia
- China
- East Timor
- Gaza Strip
- Georgia
- Hong Kong
- India
- Indonesia
- Iran
- Iraq
- Israel
- Japan
- Kazakhstan
- Korea, North
- Korea, South
- Kuwait
- Kyrgyzstan
- Laos
- Lebanon
- Macau
- Malaysia
- Maldives
- Mongolia
- Nepal
- Oman
- Pakistan
- Philippines
- Qatar
- Saudi Arabia
- Singapore
- Sri Lanka
- Syria
- Taiwan
- Tajikistan
- Thailand
- Turkmenistan
- United Arab Emirates
- Uzbekistan
- Vietnam
- Yemen
-
Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Gibraltar
- Greece
- Greenland
- Guernsey
- Hungary
- Iceland
- Ireland
- Isle of Man
- Italy
- Jersey
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Netherlands
- Norway
- Poland
- Portugal
- Romania
- Russia
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Oceania
- Home
- Products
-
Products
- Pharmaceutical Quality Dextran
- Dextran 1 (EP/USP)
- Dextran 1.5
- Dextran 3.5
- Dextran 5
- Dextran 10
- Dextran 20
- Dextran 25
- Dextran 40 (EP/USP/JP)
- Dextran 60 (EP)
- Dextran 70 (EP/USP/JP)
- Dextran 110
- Dextran 150
- Dextran 250
- Dextran 500
- Technical Quality Dextran
- Dextran T1
- Dextran T1.5
- Dextran T3.5
- Dextran T5
- Dextran T6
- Dextran T10
- Dextran T20
- Dextran T25
- Dextran T40
- Dextran T60
- Dextran T70
- Dextran T110
- Dextran T150
- Dextran T250
- Dextran T500
- Dextran T750
- Dextran Derivatives
- DEAE-Dextran 500
- DEAE-Dextran 500 Pharmaceutical Quality
- Dextran Ultra
- Dextran 10
- Dextran 20
- Dextran 40
- Dextran 70
- Dextran 500
- Dextran Standards & GPC Standards
- Dextran Kit
- Dextran 1
- Dextran 5
- Dextran 12
- Dextran 25
- Dextran 50
- Dextran 80
- Dextran 150
- Dextran 270
- Dextran 410
- Dextran 670
- Dextran 2000
- Dextran 3800
- Dextran 5400
- Application Areas
- about dextran
- PentaHibe®
Dextran 1 GPC Standard
GPC standards can be used for calibration and molecular weight determination. Critical parameters for gel permeation chromatography (GPC) standard Dextran 1 are summarized in the table below in the folder Specification.
Data Samples for GPC Standard Collection.
The files below illustrate Dextran GPC Standard 1 data and charts. The GPC Standard data are unique for a given Batch No. When ordering GPC standard from Pharmacosmos, please note that the Batch No. may be different from the examples given here.
Safety Data Sheet
GPC Standard Dextran 1
| Mp | 1,080 Da |
|---|---|
| Mw | 1,270 Da |
| Mn | 1,010 Da |
| |η| (visc.) | 4.4 ml/g |
| Mw (LALLS) | – |
| Mn (EGT) | 1,034 Da |
Where Mw is the weight average molecular weight; Mp is the peak average molecular weight; Mn is the number average molecular weight; and |η| is the intrinsic viscosity measured at 25°C.
GPC Standards
GPC Standard Dextrans from Pharmacosmos are packed and stored in safe and clean conditions.
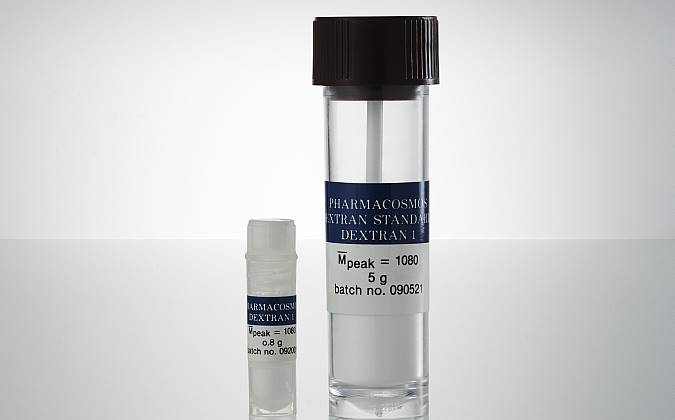
GPC Standard Dextran 1 are available in the following pack sizes:
| Item no | Weight | Packaging |
| 5522 0001 0001 | 800 mg/vial | Polypropylene plastic tube |
| 5522 0001 0002 | 5 g/vial | Polypropylene /Polyehylene plastic tube. |
Pharmacosmos supplies GPC Standard Dextrans to all countries.
Calibration and Molecular Weight Determination
GPC standards can be used for calibration and molecular weight determination
Request Quotation
Suggested reading
Approved
Pharmacosmos is the only dextran manufacturer holding certificates from the US FDA and European Directorate for the Quality of Medicines (EDQM)
Quality Delivered
Pharmacosmos delivers a unique dextran quality through a production technology that completely avoids the use of organic solvents and a quality system that meets the strictest cGMP standards for human medicines
Global Service

Pharmacosmos sells and ships directly to clients everywhere in the World. We deliver Pharmaceutical Quality Dextran of the highest standards, including those of the European Pharmacopoeia (EP), the United States Pharmacopoeia (USP), and the Japanese Pharmacopoeia (JP).

